Follicle Selection into the Preovulatory Hierarchy
In vertebrate species that ovulate a finite number of germ cells per ovulation cycle (e.g., 1 to 20 in mammals; 1 in birds and some reptiles), it is the process of ovarian follicle selection that represents the rate-limiting stage for obtaining mature oocytes. Follicle selection represents a process currently undefined in any vertebrate species by which one or a limited number of undifferentiated follicle(s) initiate final differentiation and maturation in preparation for ovulation. Accordingly, this process ultimately determines the reproductive capacity per ovulatory cycle in vertebrate species whose strategy emphasizes a limited number of offspring combined with significant parental investment (as compared to most fish and amphibians).
Unlike the estrous/menstrual cycles of mammals, the avian ovulation cycle reoccurs on an approximate daily basis until a species-specific number of eggs (a clutch) is produced. The hen (Gallus gallus) ovary is a particularly useful model for studying vertebrate follicle selection and differentiation due to the ability to accurately assess the stage of development for any growing follicle based upon size and extent of yolk incorporation. In a reproductively active hen, the five to eight largest preovulatory follicles (9-35+ mm in diameter; F1 represents the largest preovulatory follicle to be ovulated next, F2 follicle the second largest follicle, etc.) form a hierarchy and represent those that have already progressed through the process of follicle selection. By all standards, granulosa cells from preovulatory follicles are considered differentiated based upon their high-level expression of luteinizing hormone (LH) receptor (LHR), P450 cholesterol side-chain cleavage (P450scc) enzyme activity and Steroidogenic Acute Regulatory (StAR) protein, plus the ability to rapidly produce large amounts of steroids (specifically, progesterone) in response to LH. In addition, there is a larger group of slow growing, 1-5 mm follicles, and importantly, a small cohort (generally numbering 8 to 12) of 6-8 mm prehierarchal follicles1 from which one follicle per day is selected into the preovulatory hierarchy. Granulosa cells from prehierarchal follicles are mitotically active, but undifferentiated, as defined by the expression of follicle stimulating hormone (FSH) receptor (FSHR), negligible expression of LHR, and inability to synthesize progesterone. The maintenance of this cohort in a viable, but undifferentiated, state is thus a prerequisite for the daily selection of a follicle into the preovulatory hierarchy.
Characteristics Associated with Prehierarchal Follicles and Follicle Selection in the Avian Ovary
Granulosa cells (of epithelial origin) from hen prehierarchal (6-8 mm) follicles are actively maintained in an undifferentiated state, and fail to produce significant amounts of any steroid, in vitro. An inability to undergo differentiation persists despite the fact that granulosa cells express FSH receptor (FSHR) mRNA (Woods and Johnson, 2005) and readily bind FSH (which results in very low levels of cAMP formation). Granulosa cells from prehierarchal follicles also express very low levels of P450scc enzyme mRNA and protein but non-detectable activity. The inability of granulosa cells to produce progesterone is indirectly attributed to the absence of StAR expression and directly attributed to constitutively active Mitogen Activated Protein Kinase (MAPK) and protein kinase C (PKC) signaling (Woods, Haugen and Johnson, 2007). Immediately following follicle selection into the preovulatory hierarchy, in vivo, the granulosa layer begins a rapid process of differentiation that includes the transition from FSHR to LH receptor (LHR) dominance (Woods, Haugen and Johnson, 2007) and increased expression of P450scc enzyme activity due to enhanced cAMP accumulation. Subsequently, granulosa cells from preovulatory follicles produce increasingly greater amounts of basal and gonadotropin-induced progesterone as they progress through the follicle hierarchy. This high capacity for LH-induced progesterone production is prerequisite for ovulation.
The ultimate signal responsible for follicle selection is as yet undefined in any vertebrate species, and fundamental differences exist in follicle dynamics between avian and mammalian species. During an ovulation cycle in mammals there occurs a transient increase in circulating FSH over several days that initiates recruitment of a group of follicles (a follicle wave), followed by a subsequent decline in circulating FSH. Selection of a dominant follicle from this wave is predicted to occur only in the follicle(s) that express the highest levels of FSHR and aromatase enzyme, while all remaining follicles within this wave are destined to become atretic due to insufficient gonadotropin support (Fortune et al., 2001). By comparison, the avian ovulation cycle reoccurs on an approximate daily basis. Despite only minor fluctuations in circulating FSH before and after the daily preovulatory LH surge, FSHR mRNA is significantly elevated in a single prehierarchal follicle (presumably the next to be selected) (Woods and Johnson, 2005). Importantly, there is no synchronized wave of follicle atresia in non-selected prehierarchal follicles. In fact, this cohort of prehierarchal follicles must remain viable to insure the subsequent availability of follicles for selection during successive days. Such differences in follicle dynamics between the avian and mammalian ovaries suggest that there exist fundamental differences in the actions of paracrine and autocrine factors on granulosa cell gene expression and cell signaling surrounding follicle selection.
To date, we have largely focused on paracrine/autocrine factors and cell signaling pathways related to the initiation of granulosa cell differentiation immediately following follicle selection (e.g., at and subsequent to MAPK/Erk desensitization; see Granulosa Cell Differentiation). Current studies now focus on the coordination of factors that maintain prehierarchal follicle granulosa cells in a proliferative and undifferentiated state prior to follicle selection. It is reasoned that determining factors plus associated signaling and transcriptional regulatory mechanisms responsible for preventing premature differentiation will directly aid in identifying the ultimate signal for follicle selection.
Gallus Bone Morphogenetic Protein (BMP) receptors (Alks) and BMP ligands as Modulators of Granulosa Cell Differentiation in Prehierarchal Follicles
BMPs (including Growth Differentiation Factor 9; GDF-9) comprise a subfamily of the larger TGFbeta superfamily that includes previously investigated TGFbeta1, -2 and -3, plus activin A (see Granulosa Cell Differentiation). The active (cleaved) forms of Gallus BMP isoforms retain considerable homology compared to the human and mouse homologs (Table 1; Woods, unpublished).
While TGFbeta1 binds to the TGFbetaRII and RI (Alk5) receptors to activate Smad2 signaling, BMPs promote dimerization of BMPRII and RI (Alk2, Alk3, Alk6) and activate signaling via Smad1, -5 and/or -8 (Smad1/5/8) proteins. Phosphorylated (activated) Smad proteins act as nuclear transcription factors and can interact with additional co-activators and co-repressors, including promoter E-box proteins. Each of these receptors is expressed in the avian ovary.
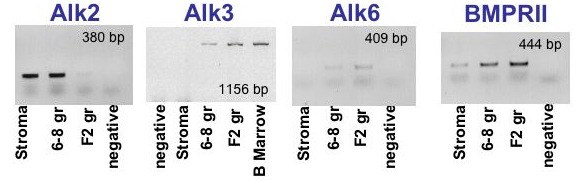
Expression of BMP receptors in the avian ovary as determined by RT-PCR and verified by sequencing. Alk2, Alk3 and Alk6 (BMPRI) receptors plus the BMPRII receptor are each expressed in undifferentiated granulosa cells (6-9 gr) from prehierarchal follicles. F2, differentiated granulosa cells from the second largest preovulatory follicle; Stroma, ovarian stromal tissue; B marrow, bone marrow; negative, negative control (no template). Unpublished; Onagbesan et al., 2003.
BMPs are recognized as multifunctional paracrine/autocrine regulators of mammalian follicle function, evidenced by the ability of BMPs to control granulosa cell proliferation and differentiation plus oocyte development. There is currently a body of literature describing the varied expression and functions of BMPs within the mammalian ovary derived from multiple species (for review, see Shimasaki et al., 2004). By comparison, there is comparatively little information regarding expression or the role of BMP family members within the avian ovary, and even less information from prehierarchal follicles. BMP-2, -4, -6 and -7 mRNA have previously been documented in both hen granulosa and theca tissues, but to date only from preovulatory follicles (Onagbesan et al., 2003). Recent studies have established that several BMPs are highly expressed within the granulosa layer from prehierarchal follicles, and that autocrine and/or paracrine produced BMPs have the potential to constitutively inhibit premature differentiation of granulosa cells. This inhibition can occur by preventing induction of LHR expression and the initiation of progesterone production.
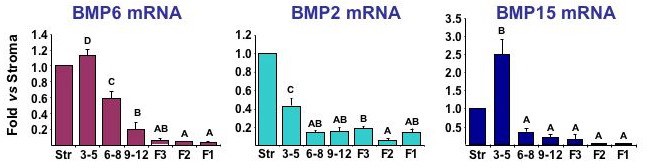
BMP2, BMP6 and BMP15 mRNA expression in granulosa layers collected from undifferentiated granulosa cells from 3-5 and 6-8 mm prehierarchal follicles, differentiating granulosa cells from the recently selected 9-12 mm follicle, and differentiated granulosa cells from the third largest (F3), second largest (F2), and largest (F1) preovulatory follicle. Data represent the mean +/- sem from three replicates compared to levels in ovarian stromal (Str) tissue. Granulosa layers from 3-5, 6-8 and 9-12 mm follicles include the oocyte. Note that highest levels for each BMP occur in undifferentiated granulosa cells from 3-5 mm follicles. Unpublished.
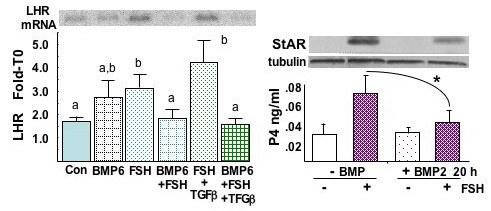
Left Panel. Representative Northern blot of LHR mRNA in prehierarchal follicle granulosa cells cultured for 20 h in the absence (Con) or presence of BMP-6 (10 ng/ml), rhFSH (100 ng/ml), and/or TGFbeta1 (10 ng/ml). Note that BMP-6 treatment prevents FSH- and FSH+TGFbeta1-induced LHR expression. a,b,c: P<0.05, by ANOVA and Fisher's multiple range test. Right panel. Pretreatment with BMP-2 (10 ng/ml) attenuates FSH (100 ng/ml)-induced StAR protein expression and progesterone (P4) production in granulosa from prehierarchal follicles. tubulin is used for standardization. * P<0.05. Unpublished.

