Initiation of Granulosa Cell Differentiation Following Follicle Selection into the Preovulatory Hierarchy
Follicle selection represents the process that ultimately determines the characteristic number of eggs to be ovulated (clutch size) by any given species. A prehierarchal follicle selected into the preovulatory hierarchy immediately begins the process of growth and final differentiation, and this is reflected by both the ability of the follicle to rapidly incorporate yellow yolk, and of the granulosa layer to initiate increasingly large (microg) amounts of progesterone production. While the ultimate signal(s) and cellular events that provide a single follicle the selective advantage to initiate rapid growth and final differentiation have yet to be described in birds (or any vertebrate), the consequent differentiation that occurs within the granulosa layer within the hen follicle immediately following selection has been the subject of recent investigations.
Among the endocrine, paracrine and autocrine factors that have been implicated in directly initiating steroidogenesis and/or enhancing gonadotropin (FSH and LH) receptor expression in granulosa cells from prehierarchal follicles are included FSH and the TGFbeta superfamily members, TGFbeta and activin. Both TGFbeta and activin signal via type I plus type II receptor-mediated phosphorylation of the Smad2 regulatory protein, and a primary action in granulosa cells is to enhance FSH receptor expression (Johnson, Bridgham and Woods, 2004). The actions of activin, but not TGFbeta, can be modulated by the presence of an activin-binding protein, follistatin.
However, evidence supports the proposal that tonic inhibition of FSH-mediated StAR, P450scc, and P45017alpha-OH expression, in vivo, renders granulosa cells from prehierarchal follicles steroidogenically incompetent. Such inhibitory signaling is also proposed to prevent any increase in FSH receptor number and to preclude premature LH receptor expression. Thus, all but one prehierarchal follicle are maintained in an undifferentiated state despite the continued, daily exposure of all follicles to circulating concentrations of FSH. In fact, FSH-induced P450scc and StAR expression plus progesterone production, in vitro, is completely blocked by EGF family ligands (including EGF, transforming growth factor-alpha and betacellulin) signaling via the mitogen activated protein kinase (MAPK)/extracellular regulated kinase (Erk) pathway. Moreover, MAPK/Erk signaling blocks TGFbeta- and activin-induced receptor signaling by preventing the phosphorylation required for Smad signaling. Significantly, inhibition of Erk signaling using the selective MAP kinase inhibitor, U0126, not only promotes a synergistic enhancement of StAR expression and progesterone production compared to FSH treatment alone, but also promotes greatly enhanced expression of the FSH receptor plus the initiation of LH receptor expression (Woods and Johnson, 2005). Accordingly, the following model depicts proposed events initiated within the granulosa layer immediately following the selection of a prehierarchal follicle into the preovulatory hierarchy.
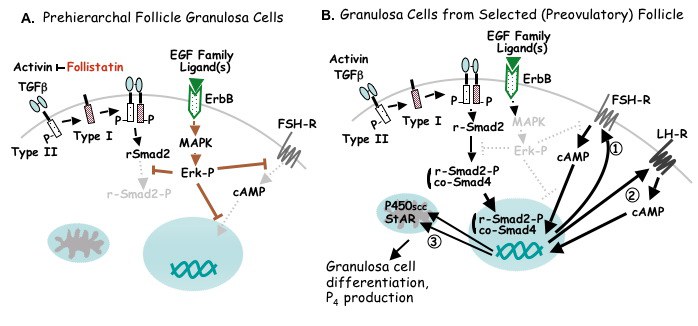
Proposed model for cellular events mediating the initiation of steroidogenesis and gonadotropin receptor expression in granulosa cells immediately subsequent to follicle selection. A. Both type I and type II TGFbeta plus activin receptors are expressed within the granulosa cell layer. While the autocrine/paracrine factor, TGFbeta, can bind to its respective receptors, the bioactivity of activin is modulated by follistatin (produced within granulosa cells). It is proposed that active induction of FSH receptor (FSH-R) expression by TGFbeta (and possibly activin) is precluded in all but one prehierarchal follicle per day by one or more EGF family ligand binding to one or more ErbB receptor isoform and the tonic activation of MAPK/Erk signaling. The sites of MAPK/Erk-mediated tonic inhibition include the absence of Protein Kinase A/cAMP signaling pathway both prior and subsequent to cAMP formation, and the prevention of Smad2 phosphorylation (Smad2-P). The absence of phosphorylation prevents transport of regulatory (r-) Smad2 to the nucleus via the co-regulatory Smad, co-Smad4. B. Following selection, a transient attenuation of MAPK signaling enables TGFbeta (and activin) signaling via Smad, and results in enhanced FSH-R expression (1). Elevated FSH-R expression subsequently facilitates FSH-induced LH-R expression (2), P450scc and StAR expression, and as a consequence, progesterone production (3). For additional details, see text and Woods and Johnson, 2005; Woods, Haugen and Johnson, 2005.
Termination of MAP Kinase Signaling
Mechanisms proposed to attenuate MAPK/Erk signaling, in vivo, in the single follicle selected into the preovulatory hierarchy per day include the rapid upregulation of Erk-specific phosphatases, including DUSP5 and MKP3.
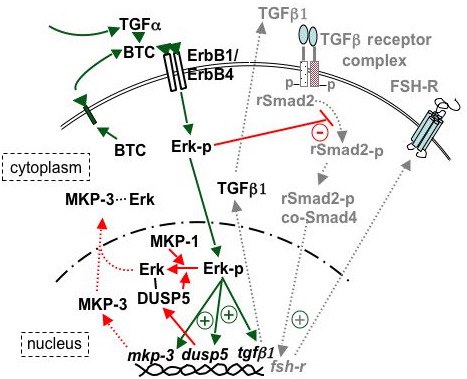
Proposed model for the role of phosphatase(s) in the termination of Erk signaling, an event prerequisite for the initiation of differentiation in hen granulosa cells. Acute effects are indicated by solid lines, while latent (MKP-3) or consequent (TGFbeta signaling) effects are indicated by dotted lines and light shading. EGF family ligands, produced in a paracrine (e.g., TGFalpha) or autocrine (e.g., BTC) fashion, bind with ErbB1 and/or ErbB4 receptors (18) to activate Erk signaling. Active Erk signaling has previously been shown to block Smad signaling (-) and TGFbeta-induced differentiation (8). Phosphorylated Erk is rapidly translocated (within 20 min) to the nucleus (Fig. 6B) where it initiates (+) the transcription of mRNA encoding TGFbeta1 (18), as well as the MAPK-selective phosphatases, DUSP5 and MKP-3 (Figs. 7B, 8B). Rapidly synthesized DUSP5 (perhaps combined with constitutive low levels of MKP-1 and MKP-3) dephosphorylates nuclear-localized Erk-p within 180 min (Fig. 6C). Induced MKP-3 occurs somewhat later (within 20 h), and is proposed to mediate the exit of Erk from the nucleus (Fig. 8C) plus affect a more prolonged desensitization of Erk signaling (Fig. 2). In turn, phosphatase activity is regulated by proteasome degradation (not depicted). Newly transcribed and translated TGFbeta1 can promote activation of TGFbeta receptor complexes (dashed lines). Following the reduction of inhibitory Erk signaling, active signaling via regulatory (r) and common (co-) Smads promotes enhanced expression of FSHR to initiate the process of granulosa cell differentiation. For additional details, see Woods and Johnson, 2006.
Gallus gallus EGF Family Ligand Orthologs
While the identity of the biologically relevant ligand(s) that promote MAPK signaling in granulosa cells has/have not been established, it has been determined that multiple Epidermal Growth Factor (EGF) family ligands are expressed within the avian ovary. All mature forms of these ligands are proteolytically shed from a membrane anchored precursor protein by one or more matrix metalloproteinases. The processed, soluble peptides each contain one conserved 3-disulfide loop motif (EGF-like domain; CX6-7CX4-5CX10-13CX1CX8-12C), yet otherwise share relatively low homology with one another. This conserved EGF motif is considered important for tertiary structure stabilization and receptor binding. Accordingly, one or more of these ligands may act in a paracrine and/or autocrine fashion to tonically inhibit granulosa cell differentiation until the time of follicle selection.
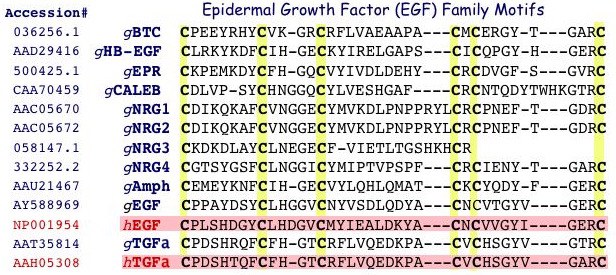
Alignment of conserved Cys residues within EGF domains from Gallus gallus (g) EGF family members currently identified from GenBank or deduced from EST databases (e.g., BTC, EPR, NRG3; www.chick.umist.ac.uk) compared to human (h) EGF and TGFalpha. BTC, betacellulin; HB-EGF, heparin-binding EGF; NRG, neuregulin; Amph, amphiregulin; TGFa, transforming groth factor alpha. With the exception of gCALEB, each of the Gallus peptides has a human ortholog, and is expressed within the hen ovary. Unpublished.
Potential for Paracrine and/or Autocrine Signaling via EGF Family Ligands
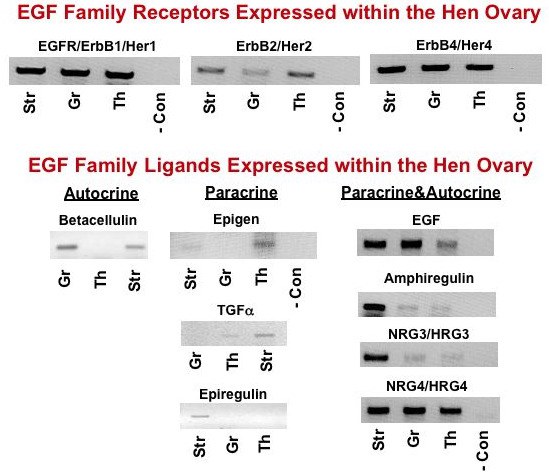
Tissue-specific amplification (by PCR) of EGF (ErbB) receptors (top panels) and EGF family ligands (bottom panels) in ovarian stromal tissue (Str) and in granulosa (Gr) and theca (Th) tissues from prehierarchal follicles. Collectively, these results provide evidence for potential autocrine and/or paracrine signaling to promote tonic MAPK within undifferentiated granulosa cells prior to follicle selection. -, negative control (no template). From: Woods, Haugen and Johnson, 2005 and Unpublished.
MAPK/Erk signaling by EGF family ligands in hen granulosa cells
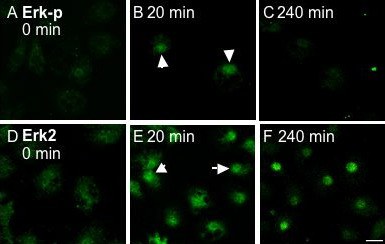
Cellular localization of Erk-p following treatment of granulosa cells without (0 min) or with 25 ng/ml TGFalpha for 20 or 240 min. A-F, Granulosa cells were processed for confocal microscopy and immunofluorescently labeled for endogenous Erk-p using FITC conjugated secondary antibodies (top row; A-C) and Erk2 (bottom row; D-F). Images were taken across a single confocal plane at the cell body (x/y axis). A-C, Following treatment with TGFalpha, endogenous Erk is phosphorylated and translocated to the nucleus of all cells by 20 min (B, arrows), and is subsequently dephosphorylated by 240 min (C). This pattern of transient phosphorylation parallels that established by Western blot analysis (Fig. 1A). D-F, Erk2 is present throughout the cytoplasm prior to TGFalpha treatment (D), is translocated to the nucleus following a 20 min treatment with TGFalpha (E, arrows), and remains localized within the nucleus after 240 min (F). Nuclear localization was verified by counter staining with Draq5 (not shown). Bar = 10 mm. From: Woods and Johnson, 2006.
Gallus TGFbeta Orthologs, and Expression of TGFbeta Ligand plus Receptors in the Vertebrate Ovary
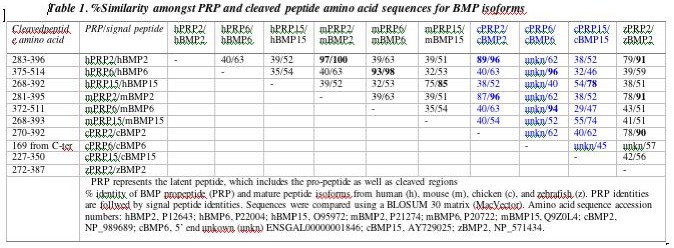
Percent identity for full-length Latent Active Peptide (LAP) sequences / processed, active peptide sequences of TGFbeta isoforms among various vertebrate species. The chicken (c) TGFbeta4 isoform is now known to represent the ortholog to human (h) TGFbeta1. m, mouse; x, xenopus. Woods, unpublished.
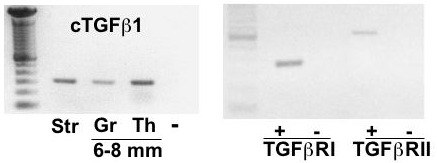
Left panel: Amplification of chicken TGFbeta1 cDNA from ovarian stromal (Str) tissue, and granulosa (Gr) and theca (Th) tissue from prehierarchal (6-8 mm) follicles by PCR. Right panel: Amplification of TGFbeta RI (Alk5) and RII cDNA from granulosa cells collected from prehierarchal follicles. Left lane represents DNA size markers in multiples of 100 bp; - : represents the negative control (absence of reverse transcribed template). From: Woods et al., 2005.

